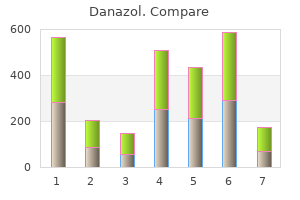
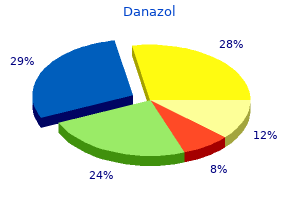
Danazol
"Cheap 100 mg danazol with amex, menopause periods".
By: P. Mufassa, MD
Medical Instructor, Tufts University School of Medicine
The first step is an N-O acyl shift at the intein Nterminus women's health diet tips generic danazol 100 mg with amex, followed by a transesterification reaction that results in forming the branched intermediate menopause 33 order danazol online from canada. The intein is cleaved from the branched intermediate by cyclization of the intein C-terminal Asn menopause zaps buy danazol 50mg overnight delivery. The native peptide bond is formed between the two exteins after a spontaneous O-N shift pregnancy effacement order generic danazol from india. Mutagenetic data indicate that the penultimate His residue of the intein is required for branch resolution (succinimide formation) and thus may be involved in deprotonating the intein C-terminal Asn, directly or via a charge relay system. However, these residues do not necessarily have to be adjacent to the Asn in primary amino acid sequence, but they need only be correctly positioned in the three-dimensional structure of the enzyme. It has been proposed that the conserved His in Block B assists in the acyl shift at the N-terminal splice junction (20). The identification of all the residues involved in these nucleophilic attacks awaits further mutagenetic experiments and the determination of the crystal structure of a splicing precursor. Even conservative substitutions of the splice junction Ser, Thr, or Cys residues result in reduced or blocked splicing. When the splicing reaction is perturbed by conservative mutation or expression of the intein in unacceptable foreign protein contexts, the most commonly observed phenomenon is cleavage at one or both splice junctions in the absence of extein ligation. C-terminal cleavage occurs when Asn cyclization predominates or precedes branch formation. N-terminal cleavage occurs when the upstream ester bond is hydrolyzed or attacked by other nucleophiles before branch formation. Intein N-terminal cleavage and autocleavage of several proteins, including glycosylasparaginase (27) and the hedgehog protein (28), proceed essentially by the same pathway and have an acyl shift resulting in ester (thioester) formation at a Ser, Thr, or Cys, followed by cleavage via nucleophilic attack on the resultant ester (or thioester). Protein Splicing and Protein Engineering Understanding the protein splicing pathway opens up avenues for protein engineering. For example, mutating the C-terminal splice junction Asn and Ser (or Thr/Cys) results in inteins that fail to splice but instead cleave the N-terminal splice junction. Likewise, mutating the intein N-terminus (Ser, Thr, or Cys) results in inteins that cleave only at their C-terminal splice junctions. Furthermore, inteins may provide a new method for controlling expression of active proteins. The target protein is inactivated when an intein is present, but it can be activated by splicing the intein. For example, cold-sensitive mutants of the thermostable Psp pol intein-1 splice at temperatures over 30°C, but not below 30°C. Temperature-sensitive mutants of inteins from mesophiles splice at low temperatures but not higher. Removable blocking agents incorporated into essential junction residues prevent splicing until they are removed (30). Controllable splicing elements provide a means of expressing toxic proteins or making controllable knockouts. Finally, modified inteins provide a means of introducing electrophilic esters and thioesters into proteins, which then can be exploited to tag proteins with nucleophilic reagents (fluorescent labels, radioactive Cys residue, peptides) at their C-termini or as substrates for protein semisynthesis (31). For proteins from Protein Stability Protein stability involves the ability of the native, folded protein structure to withstand the disruptive, denaturing influence of the external environment, its resistance to unfolding (see Proteins). This depends on the type of disruptive influence (eg, extremes of temperature, pressure, pH, or concentration of denaturants) and on the criteria for judging the nativeness of the folded conformation. The criteria might be a specific functional property of a given protein (eg, catalysis, ligand binding, etc. However, when protein denaturation (unfolding) is a highly cooperative process (see Proteins), all of these changes occur simultaneously with variation of the denaturing conditions. In that case, the stability of a protein against any external factor can be specified by the value of the factor that induces 50% of the maximal change in the protein properties. These semiquantitative measures of protein tolerance to various factors have practical importance for specifying the range of external conditions in which that protein preserves its native structure and functions.
The latter has a core of b-sheet consisting of nine bstrands describing a 180° right-handed twist flanked by helical segments women's health center buffalo ny generic 50 mg danazol amex, loops menopause blood test order 100mg danazol otc, and some additional b-strands women's health problems in sri lanka buy danazol 50mg cheap. Because binding here is some 20-fold tighter than at the active site menopause symptoms buy danazol paypal, it is suggested that phosphorylase is anchored by these sites to a glycogen particle, whether or not it is acting on other glycogen end-chains at the active site. Components of the active site and the inhibitor site for caffeine (Phe285) are found at the interface with the C-terminal domain. The latter domain contains a core of six parallel b-strands, with a twist and connectivity to surrounding a-helices that is topologically similar to the nucleotide-binding motif of the dehydrogenases, although more buried by additional helices and loops. The dimer possesses a concave catalytic face containing the active sites and glycogen storage sites, by which it may bind to glycogen while exposing the convex control face to the action of phosphorylase kinase or phosphatase. Space-filling model of the control face of the phosphorylase a dimer viewed down the twofold axis. Positively charged residues are blue, negatively charged residues are red, and the phosphate groups of the two Ser14 residues are magenta, while noncharged residues of the N-terminal a-helices are yellow. The N-terminal segments, identified by the phosphates and yellow residues, lie across the subunit interface. This T-state structure, with the phosphates fully exposed and surrounded by positive charges, represents the favored substrate for protein phosphatase. Studies on the catalytic mechanism of phosphorylase have been reviewed up to 1985 (3) and centered around the role of pyridoxal 5-phosphate. The coenzyme, sandwiched between the two domains, plays an unusual structural role because its removal leads to an inactive monomer. Extensive analog studies have eliminated all parts of the coenzyme from participating in catalysis except the phosphate, which must be capable of forming a dianion. Furthermore, pyrophosphate showed competitive inhibition with both glucose-1-P and the activating phosphite, while binding only one mole per monomer. That same year, crystallographic studies placed only the phosphate of the coenzyme near the substrate, 7. These results led to the interacting phosphates hypothesis that was strengthened by a number of experiments, including the reconstitution of the enzyme with pyridoxal pyrophosphate glucose. This compound contains both substrate and coenzyme covalently linked, and the enzyme could transfer the glucose to an added oligosaccharide. This suggested that the coenzyme phosphate might act as an electrophile (Lewis acid). However, the alternative hypothesis that the coenzyme phosphate acts as a proton donor (Brшnsted acid) now appears more likely, especially considering the crystallographic studies of time-resolved catalysis in the crystalline state (4). Phosphorylase catalyzes the phosphorylation of heptenitol to heptulose 2-phosphate, and the latter, a potent inhibitor, is found in the crystal structure with a hydrogen bond between its phosphate and that of the coenzyme. The phosphate anion stabilizes the glucosyl carbonium ion, necessary to maintain the alpha anomeric configuration. The reaction is completed by a nucleophilic attack of the phosphate on the carbonium ion to form glucose 1-phosphate. Regulation the two forms of the enzyme differ markedly in their allosteric regulatory properties. Glucose binds at the active site of phosphorylase and is a competitive inhibitor of glucose-1-P, a primitive end-product feedback inhibition control. Phosphorylase a may be said to have "escaped allosteric control," the latter form of regulation having been superseded by covalent modification, under extracellular hormonal and neural regulation. Comparison of the two T-state tetragonal crystal structures reveal that the disordered N-terminal segment, residues 516, of the b form becomes an ordered helix which lies across the dimer interface in the a form, with eight new intersubunit hydrogen bonds between polar groups, plus additional hydrophobic interactions. The tighter subunit associations of the dimer in the a form account for it obeying the allosteric concerted model of Monod et al. Thus the structural studies of phosphorylases a and b have resolved an old controversy within a single enzyme. Withers (1986) In Vitamin B6 Pyridoxal Phosphate, Part B, John Wiley & Sons, New York, pp. More than a dozen different proteincarbohydrate linkages have been identified in glycoproteins, and they are often categorized as either O-glycosidic (O-glycans or O-linked oligosaccharides), in which carbohydrate is covalently attached to protein via a hydroxyl group in the side chains of several amino acids, or N-glycosidic (N-glycans or N-linked oligosaccharides), in which sugar is covalently attached to protein through the amide nitrogen of asparagine residues. Protein glycosylation is the most diverse type of post-translational modification of proteins. The biochemical processes through which these linkages are generated are commonly called Oglycosylation and N -glycosylation pathways, and each pathway involves dozens of different enzymes. There are dramatic differences between these pathways in terms of their subcellular localization, the enzymes involved, the structures of the oligosaccharides synthesized, and the biological functions the glycans.
Purchase discount danazol on line. TOP HEALTH TIPS FOR WOMEN | Pamela Popper Ph.D N.D..
This is normally sufficient for the proteinase to cleave susceptible peptide bonds pregnancy myths discount danazol 200mg on-line, which typically generates 10 to 20 fragments of 50- to 70-kDa proteins (1) womens health 95825 discount danazol 50 mg mastercard. Suitable enzymes are trypsin pregnancy 3 weeks symptoms discount danazol 200 mg free shipping, a-chymotrypsin women's health clinic uihc discount danazol 200mg on line, and papain, in addition to the popular V8 proteinase from Staphylococcus aureus. All are added in proportions corresponding to an enzyme to substrate ratio in the range of 1:10 to 1:50 (w:w). After digestion, electrophoresis is resumed, and the resulting fragments are resolved, typically in a 15 or 20% acrylamide gel of the discontinuous type. A unique proteolytic pattern is obtained for each protein substrate and for each individual proteinase. This discriminating feature makes Cleveland mapping useful as a tool for visually judging the similarity between proteins. In addition to enzymes, specific chemical cleavage is employed where the protein substrate within a gel is treated in a test tube. Suitable reagents are cyanogen bromide for cleavage after methionine residues, N-chlorosuccinimide for cleavage at tryptophan, hydroxylamine for cleavage at Asn-Gly peptide bonds, and formic acid for cleavage at Asp-Pro bonds. As for the size of the protein substrates, the method is generally applicable to polypeptide chains in the range 10 to 100 kDa. This can be useful also for proteins large than 10 kDa in order not to waste small fragments (5). Clonal Selection Theory At the beginning of the twentieth century, Ehrlich was the first to propose that antibodies did exist prior to the introduction of antigen or immunogen and that antigenic stimulation only promoted amplification of molecules of the appropriate specificity. This proposal was quite pertinent, because it contained several key ideas, proven correct some 70 years later. One is the selective process of a preexisting repertoire, the other is that immunoglobulins (Igs) may exist as surface or secreted molecules. The only aspect that was not verified is that Ehrlich imagined a cell to express many different antibodies. After a long period during which hapten analysis indicated the exquisite precision of antibodyantigen interactions, it seemed impossible that antibody structures might preexist to recognize laboratory artifacts, like the haptens being studied. This resulted in the template theory of antibody formation, which required the presence of antigen prior to the synthesis of the corresponding antibodies. Revival of the selection theory was proposed in 1955 by Jerne, but it took another 4 years before the final form of the "clonal selection theory" was put forward by Burnet. The essence of the theory was that antibody specificities were preformed, but in a clonally distributed manner, so that one cell makes only one antibody of one given specificity. This was crucial to provide a mechanism accounting for acquisition of discrimination between self and nonself, after the observation on induction of immunological tolerance by the group of Medawar in 1953,when it was demonstrated that tolerance was an acquired phenomenon, resulting from a contact between the immature immune system and the self antigens during gestation or the perinatal period. Burnet most simply explained tolerance by a deletion of those clones that could interact with self components, leading to the concept of "forbidden clones. Many investigators worked to define models that would prove the clonal theory, and the literature is amply documented on this point, with many very elegant approaches, such as those based on limiting dilutions, either in vitro or in vivo after transfer in irradiated mice. The ultimate proof came from molecular analysis, first at the protein level on myeloma proteins and then when the mechanism of gene rearrangement of Ig genes was elucidated, under the control of allelic exclusion. Some exceptions to the clonal expression of Ig molecules have been periodically reported. Upon reactivation of the recombinase genes upon immunization, for example, a transient coexpression of different V genes may occur, leading to secondary gene rearrangements. But this rarely results in long-term production of antibodies of different specificities. The clonal theory is widely accepted and considered proven, and the fantastic use of monoclonal antibodies is (for most biologists) daily living proof of its pertinence. See also entries Allelic Exclusion, Antibody, Gene Rearrangement, and Immunoglobulin. For example, it is possible to clone cells, that is, to cause cells to reproduce themselves so as to make a population of identical cells. Gene cloning is the process of identifying and isolating a specific gene of interest. Cloning genes is a major focus of molecular biology, and the ability to clone genes has revolutionized biology.
Most minigenes are made from eukaryotic (especially mammalian) genes because of their large size women's health ketone advanced purchase 200mg danazol with visa. In most cases menopause 48 years old purchase 50mg danazol fast delivery, a sequence from the 5-end of the gene is joined to a sequence near its 3-end to form the minigene breast cancer jobs discount 50mg danazol mastercard. The smaller size of a minigene facilitates studies of its regulatory sequences (see Gene regulation) menopause 49 buy generic danazol 200mg. It is necessary to show that the behavior of the minigene resembles that of the intact gene because internal sequences affect gene regulation (1). Such a minigene has been used to obtain high-level expression of proteins in salivary glands (2). A minigene with an intact coding region has been used to introduce mutations at specific sites in the mouse genome (3). Based on this, a general function of minisatellites may be to provide binding sites for recombination proteins in eukaryotes. In the vertebrate genome, the short (10- to 50-bp) tandem, direct, repeat motifs of minisatellites contain variants of a common core sequence. Interest in them comes from the fact that the number of repeats of a given minisatellite at a certain position in the genome varies from one individual to another. Thus a probe for a particular minisatellite is highly polymorphic and reveals a locus with a great number of alleles that have a high probability of being genetically informative in the great majority of cases. But, the real solution has been provided by the discovery of microsatellites, named by analogy to the minisatellites. There are eight such base pairs and, if the directionality of the duplex is taken into account, the number of mismatches is 12. These include the insertion/deletion of single nucleotides, loops consisting of unpaired nucleotides in one strand, and bubbles, which consist of more than one mismatched base in a row. Mismatches also arise during recombination between alleles of the same gene or between closely related sequences. If these mismatches occur in meiotic chromosomes and remain uncorrected, they can give rise to anomalous segregation of the alleles, or they may lead to gene conversion upon correction. Cytosines are often methylated at the C5 position, and these 5-methylcytosines deaminate about 103-fold faster than does cytosine (1). The extent of methylation varies across species, ranging from a virtually undetectable level in yeast and Drosophila, to 1% in Escherichia coli. In humans, 20% of cytosines are methylated; consequently, G-T mismatches arising from deamination of cytosine are a common occurrence in humans. The general mismatch repair system is the most important, and is the system most widely distributed in the biological world. It has been found in all bacteria tested, as well as in model eukaryotic organisms, such as yeast, fruit flies, and humans. General Mismatch Repair Systems this repair system corrects all mismatches and small insertion/deletion loops in an essentially sequence-independent manner (2). The general reaction scheme is as follows: A nick is made 5 or 3 to the mismatch in the newly synthesized strand, which is by definition the "wrong" strand. A 5 to 3 or a 3 to 5 exonuclease digests the nicked strand, starting at the nick and terminating at some distance past the mismatch. Concomitant with exonucleolytic degradation, repair synthesis takes place, filling the gap generated by the exonuclease with correct nucleotides. The reaction mechanism of general mismatch repair has been elucidated in considerable detail in E. In addition, the Dam methyltransferase plays a crucial role in strand discrimination. A mutation in any of the mismatch repair-specific genes (ie, mutS, mutH, mutL) or in the specific methylation gene dam increases both the spontaneous and damage-induced mutation frequency by 103- to 104-fold. A nick 3 or 5 to the mismatch in the newly synthesized strand marks that strand for correction. In humans, the mechanism of preferential nicking of the newly synthesized strand is not known. The mismatch is recognized by a MutS homologue, and a MutL homologue coordinates nicking with mismatch recognition. The MutSa is involved in recognition/repair of simple mismatches and small, one- to three-nucleotide insertion/ deletion mismatches.