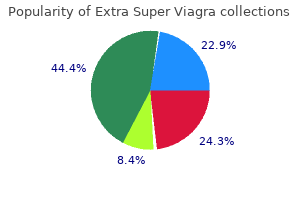
Extra Super Viagra
"Buy extra super viagra american express, best erectile dysfunction doctor in india".
By: R. Mitch, M.S., Ph.D.
Assistant Professor, Marian University College of Osteopathic Medicine
Unfortunately impotence causes cheap extra super viagra uk, taken alone erectile dysfunction drugs in bangladesh purchase 200 mg extra super viagra with mastercard, the claim language does not indicate the intended meaning of the term doctor who treats erectile dysfunction discount generic extra super viagra canada, and thus the Court must turn to the specification for guidance impotence is the best 200 mg extra super viagra. The "Abstract" states that the invention provides a "lower incidence of nausea and vomiting than conventional tablets. They then state that vomiting occurs in approximately seventeen percent of patients using the immediate release version. Thus, the inventors describe the problem in terms of the number of patients experiencing the side effects, rather then in the context of the severity of those side effects. The inventors then describe the positive effect of the extended release version of the drug in the "Brief Description of the Invention," explaining that, during clinical trials of extended release venlafaxine hydrochloride, "the probability of developing nausea" greatly reduced after the first week. Beginning in the "Brief Description of the Invention," the inventors use an additional term that is not used anywhere else in the patent. Wyeth asserts, and the Court agrees, that the term "level" implies "degree" or "severity" rather then "number. Wyeth therefore argues that the inventors intended to associate both types of reductions with the new invention. It further asserts that, because the terms "level" and "incidence" are both used to modify "nausea," the inventors clearly intended the words to be used interchangeably. This interpretation is supported by the fact that the inventors never refer to the "level of emesis," but instead - 762 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z only discuss the "incidence of emesis. Consequently, the Court must find meaning in their decision to use only the term "incidence" in the body of the claims. Moreover, because claim terms are presumed to be used consistently throughout a patent, see Phillips, 415 F. Finally, as Mylan points out, the fact that the specification indicates the "level" of nausea may be reduced by the extended release formulation does not require the Court to read that limitation into the claims. For these reasons, the Court construes the term "diminished incidences of nausea and emesis" to mean "a decrease in the number of patients suffering from nausea and vomiting compared to patients receiving the same total daily dose of an immediate release formulation that is administered at least twice a day. Specifically, Wyeth contends that "diminished incidences of nausea and emesis" means "the degree and/or frequency of nausea and emesis from the extended release formulation administered once a day is less than what would be experienced by patients receiving the same total daily dose of an immediate release formulation that is administered at least twice a day. Thus, Impax contends that the "diminished incidences of nausea and emesis" means "a decrease in the number of patients suffering from nausea and vomiting compared to patients receiving the same total daily dose of an immediate release formulation that is administered at least twice a day. Accordingly, the Court must turn to the claim language and the specification for instruction regarding the meaning of the phrase "diminished incidences of nausea and emesis. However, the specification discusses the effect of the invention on nausea and emesis in three specific areas. First, the Abstract of the invention discloses that the invention "provides a lower incidence of nausea and vomiting than conventional tablets. The Background of the Invention discusses nausea and emesis in the context of the immediate release tablets using a numerical focus. Specifically, the Background of the Invention explains that "[w]ith the plural daily dosing regimen, the most common side effect is nausea, experienced by about forty five percent of patients under treatment with venlafaxine hydrochloride. The Brief Description of the Invention contrasts the clinical advantages of the invention with the disadvantages of multiple daily dosing and discusses nausea and emesis in more general terms, as follows: the use of the one-a-day venlafaxine hydrochloride formulations of this invention reduces by adaptation, the level of nausea and incidence of emesis that attend the administration of multiple daily dosing. Thus, in accordance with this use aspect of the invention there is provided a method for reducing the level of nausea and incidence of emesis attending the administration of venlafaxine hydrochloride which comprises dosing a patient in need of treatment with venlafaxine hydrochloride with an extended release formulation - 763 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z of venlafaxine hydrochloride once a day in a therapeutically effective amount. Reviewing the specification as a whole and in context, the Court concludes that the inventors did not intend to limit the term "diminished incidences of nausea and vomiting" to a numerical or percentage based definition. Interestingly, that portion of the specification that refers to specific percentages does not even use the term "incidences," which suggests to the Court that the inventors did not necessarily intend to equate the term "incidences" with percentages or numbers. For example, the Abstract discusses the "lower incidence of nausea and vomiting" while the Brief Description of the Invention refers to "reducing the level of nausea and incidence of emesis. Indeed, the Court agrees with Wyeth that if the inventors intended to maintain a strictly numerical focus with respect to the "diminished incidences of nausea and emesis" the claim language would have used a term more commonly connected to numerical values such as "fewer incidences of nausea and emesis" or would have alternatively linked the claim language more specifically to a decreased percentage or number of patients suffering from nausea and emesis. Instead, the term "diminished" suggests the broader concept of a reduction in size, number and degree. The specification then goes on to disclose one embodiment that withstands "oxidative, hydrolytic, and cyclization degradation. Additionally, the parties previously stipulated that "discoloration" referred to oxidative discoloration. Affymetrix originally argued that "discrete known regions" are physically distinguishable and known regions. However, during its rebuttal argument, Affymetrix embraced the definition, provided in the specification, that "region" is a localized area on a surface which is, was, or is intended to be activated for formation of a polymer.
This construction encompasses mixtures that produce polyurethane foams that are made flexible upon crushing erectile dysfunction treatment natural remedies purchase extra super viagra 200mg with visa, such as the mixture disclosed in the Eling reference erectile dysfunction treatment in bangalore order extra super viagra 200mg with mastercard. Use of the term "all" supports the proposition that the aforementioned industrial compositions include the compositions of Eling erectile dysfunction caused by statins discount extra super viagra 200 mg. Additionally iief questionnaire erectile function trusted extra super viagra 200 mg, in the sentence bridging pages 7 and 8 of the specification, [Buszard] disclose[s] that "flexible polyurethane foam compositions can be made according to the present invention by reacting an isocyanate with a polyol in the presence of a foam-forming agent and a blend of tetra-halophthalate esters and phosphorus-containing flame retardant additives. On appeal, Buszard alleges-and the majority appears to agree-that the term "flexible polyurethane foam reaction mixture" has a specific meaning to one of ordinary skill in the art. Indeed, he boldly asserts in his reply brief that he "suffer[s] no duty to define an art recognized term. Moreover, although Buszard asserts that the term "flexible polyurethane foam reaction mixture" has a well-defined meaning to someone skilled in the art, not only has Buszard failed to provide any evidence to back up this assertion, his briefs also fail to tell us what that meaning is. The only enlightenment Buszard provides regarding the meaning of "flexible polyurethane foam reaction mixture" is a single page from the Kirk-Othmer Encyclopedia of Chemical Technology, which generally describes the differences between "flexible foam" and "rigid foam. According to Buszard, we should interpret the disputed claim term narrowly in light of his specification, which describes mixtures of ingredients that produce flexible foam without a crushing step. Buszard also points out that the flexible foams described in his specification are consistent with the general description of "flexible foam" in the Kirk-Othmer Encyclopedia of Chemical Technology. But whether Buszard can provide descriptions of flexible foam that he believes are consistent with his desired interpretation is beside the point. If Buszard seeks a specific claim interpretation, he should amend his claim so it conveys his intended meaning. Roxane contends that in all claims "flocculated suspension" means "a suspension of uniformly dispersed solid matter, in which the solid matter forms an open network aggregate with many branch points in the primary structure which prevents individual floccules from approaching each other closely, the open network aggregate, over time, forming a loosely packed sediment with scaffold-like structure, and not a solid cake. Roxane contends that in all claims the term "stable," which modifies the phrase "flocculated suspension," means "the flocculated suspension, upon sedimentation after storage at 40 [degrees] c and 75% relative humidity for a period of three months, can be shaken to reform the original, uniformly dispersed, flocculated suspension. Under Markman, of course, claim construction presents a question of law to be resolved by the court. Rather, [it] must look at the ordinary meaning in the context of the written description and the prosecution history. By way of further guidance, the Court suggested that "[o]ther claims of the patent in question, both asserted and unasserted, - 895 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z can also be valuable sources of enlightenment as to the meaning of a claim term. Because claim terms are normally used consistently throughout the patent, the usage of a term in one claim can often illuminate the meaning of the same term in other claims. Differences among claims can also be a useful guide in understanding the meaning of particular claim terms. For example, the presence of a dependent claim that adds a particular limitation gives rise to a presumption that the limitation in question is not present in the independent claims. Despite the importance of the specification the Court referred to Texas Digital Systems, Inc. Furthermore, like the specification, the prosecution history was created by the patentees in attempting to explain and obtain the patent. Because dictionaries, and especially technical dictionaries, endeavor to collect the accepted meanings of terms used in various fields of science and technology, those resources have been properly recognized as among many tools that can assist the court in determining the meaning of particular terminology to those of skill in the art of the invention. As a cautionary note, the Federal Circuit stated that "[w]e have viewed extrinsic evidence in general as less reliable than the patent and its prosecution history in determining how to read claim terms. In sum, extrinsic evidence may be useful to the court, but it is unlikely to result in a reliable interpretation of the patent claim scope unless considered in the context of the intrinsic evidence. As the Court explained, "[t]he sequence of steps used by the judge in consulting various sources is not important; what matters is for the court to attach the appropriate weight to be assigned to those sources in light of the statutes and policies that inform patent law. Stable Flocculated Suspension: Putting aside for the moment the meaning that Roxane attributes to "stable," the meaning of "flocculated suspension" will be addressed. Suspensions are important when formulating drugs that are insoluble in conventional solvents such as water. The problem, such as that addressed in the patents in issue, is to prevent the drug particles when they settle from forming a hard cake that cannot be redispersed. The patents at issue solve this problem with respect to megestrol in the following manner: Individual solute molecules do not bond tightly to form a cake in a flocculated suspension due to the fact that they form an open network aggregate with many branch points in the primary structure which prevents individual floccules from approaching each other closely. Flocculated suspensions have a high sedimentation height, due to the natural tendency for - 896 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z an open network aggregate to not form a cake.
Claim Construction the operative claim language of claim 7 is "consisting essentially of erectile dysfunction cause of divorce order extra super viagra 200mg on line. Ivax urges a construction of claim 7 excluding the eight adjuvants erectile dysfunction treatment blog discount extra super viagra 200mg overnight delivery, basing that on the patent specification and prosecution history problems with erectile dysfunction drugs extra super viagra 200 mg fast delivery. Warner-Lambert further argues that "adjuvant" refers to ingredients that are intimately mixed with the gabapentin erectile dysfunction treatment spray generic extra super viagra 200 mg free shipping, and not those used solely for purposes of encapsulation. Now, having had an opportunity to consider the record in more depth, the Court concludes otherwise. Findings of fact and conclusions of law made in the context of a preliminary injunction, let alone observations, do not foreclose conclusions to the contrary at later stages of the litigation. This distinction is extremely important as the BioGran (R) product indisputably contains particles which are smaller than 355 [mu]m regardless of whether traditional sieving or scanning electron microscopy is utilized to determine particle size. Plaintiffs contend that this language is intended to allow the presence of items which do not materially affect the basic characteristic, or "essence" of the composition. With regard to the language of Claim 1, the Court concludes that in order to be infringing, a composition must, at a very minimum, contain glass particles within the cited particle size range; however, the claim language does not preclude the existence of other materials which do not materially affect the essential characteristics of the composition of Claim 1. Yet the question remains, can those "other materials" be particulate glass with particle sizes below 355 [mu]m? In order to answer these questions, the Court must determine what the essential characteristics of the claimed invention are. These characteristics are based upon the improved performance of the claimed invention over prior art in the same field. The patent specification suggests that the most advantageous composition is one with the widest range of particle sizes, i. The improved performance derives from the use of particles in the ranges cited in Claim 5, i. It, therefore, seems apparent that the basic characteristics of the claimed invention are inextricably intertwined with the degree of performance associated with the use of particles within the cited ranges. Defendants do agree that the smaller particles which are present in the BioGran (R) product affect the performance of the composition, but unlike Plaintiffs, they contend that the marked difference in performance brought about by the inclusion of a greater percentage of smaller particles does materially alter the characteristics of the composition at issue. Accordingly, the Court concludes that a larger presence of particles smaller than the range cited in Claim 1, especially where the percentage thereof is greater than the onethird fraction cited in Claim 5, could easily lead to the conclusion that the basic characteristic of the claimed invention would be altered by the variance in performance brought about by their inclusion. Indeed, were a hypothetical composition to be comprised of more than two-thirds of its particles below 355 [mu]m, it would be difficult to argue that the composition consists "essentially of" particles within the range of 355 to 710 [mu]m. Dependant Claim 2 requires the composition of Claim 1 wherein 2/3 of the particles are between 300 and 360 [mu]m, and dependant Claim 3 requires a composition of Claim 2 wherein at least 90% - 625 - Jump to: A B C D E F G H I J K L M N O P Q R ST UVW XY Z of the particles are between 300 and 360 [mu]m. Thus, Claim 1 is amenable to infringement actions against compositions which not only contain particles within the size range of 355 to 710 [mu]m recited in Claim 1, but which also contain particles with sizes smaller than 355 [mu]m. Additionally, a dependant claim shall specify a further limitation on the subject matter of the claim to which it is referenced. Dependant Claim 4 adds a limitation to independent Claim 1, by requiring not only the presence of particles in the size range contained in Claim 1, but also the presence of particles in the 90 to 350 [mu]m range. Claim 4 further requires that the particles within the 355 to 715 [mu]m range of Claim 1 be comprised of particles from the two more specific ranges already claimed in dependant Claims 2 and 3, i. Plaintiffs contend that, by implication, Claim 1 must be sufficiently broad to accommodate the further restriction of dependant Claim 4. Indeed, the Patent Examiner specifically approved of the revised text of Claim 1 knowing that the dependant Claim 4 was based upon it. In doing so, the Examiner implicitly found that the scope of Claim 1 does not exclude the presence of particles smaller than 355 [mu]m. It is axiomatic in patent law that all patent claims, be they independent or dependant in nature, are presumed to be valid regardless of the validity of other claims. Furthermore, the presumption of validity of patent claims is based upon the presumption of administrative correctness associated with the actions of an agency which is charged with patentability evaluation. The Court concludes that based upon the claim language as interpreted by the Patent Office, the presumptively valid dependent Claim 4 could not exist but for the amenability of Claim 1 to the inclusion of compositions that contain particles below 355 [mu]m, so long as the composition in question also contains those essential particle sizes in the range of 355 to 710 [mu]m. Claim 1 is sufficiently broad to include particle sizes smaller than 355 [mu]m, especially when, as required by Claim 4, the smaller particles co-exist in a mixture with particles that have sizes in the range of 355-500 [mu]m, and with particles that have sizes in the range of 500-710 [mu]m.

Syndromes
- Echocardiography
- Boys as young as age 9 can receive the vaccine if their doctor recommends it.
- The amount swallowed
- Urine cortisol test
- Finger movement
- Fewer wet diapers than usual
- Keep having sex.
- Azithromycin
- Tear or rupture of the wall (septum) between the left and right ventricles (lower heart chambers)
One carbon unit (red ring) is attached to N5 and N10 groups (blue rings) of tetrahydrofolic acid Causes for Folate Deficiency Folic acid deficiency is very common in India erectile dysfunction medication free samples order extra super viagra discount, and is perhaps the most commonly seen vitamin deficiency erectile dysfunction hiv extra super viagra 200mg lowest price. Pregnancy: Folate deficiency is commonly seen in pregnancy erectile dysfunction injections videos discount extra super viagra, where requirement is increased erectile dysfunction treatment australia purchase discount extra super viagra line. Defective absorption: In sprue, celiac disease, gluten induced enteropathy, resection of jejunum and short-circuiting of jejunum in gastroileostomy, absorption is defective. Gastrointestinal enzymes in the gut remove the glutamate residues and only the mono-glutamate form of folic acid is absorbed. Anticonvulsant drugs (hydantoin, 402 Textbook of Biochemistry; Section D: Nutrition dilantin, phenytoin, phenobarbitone) will inhibit the intestinal enzyme, so that folate absorption is reduced. Hemolytic anemias: As requirement of folic acid becomes more, deficiency is manifested. Dietary deficiency: Absence of vegetables in food for prolonged periods may lead to deficiency. When B12 is deficient, this reaction cannot take place, leading to folate deficiency (see under vitamin B12). Very rapidly dividing cells in bone marrow and intestinal mucosa are therefore most seriously affected. This asynchrony or dissociation between the maturity of nucleus and cytoplasm is manifested as immature looking nucleus and mature eosinophilic cytoplasm in the bone marrow cells. The peripheral blood picture in folate deficiency is described as macrocytic, and in cobalamin deficiency as megaloblastic. Homocysteinemia Folic acid deficiency may cause increased homocysteine levels in blood. Plasma homocysteine levels above 15 micromoles / L is known to increase the risk of coronary artery diseases. Providing adequate doses of pyridoxine, B12 and folic acid may lower the homocysteine levels. Birth Defects Folic acid deficiency during pregnancy may lead to homocysteinemia and neural tube defects in the fetus. So, supplementation of folic acid from early pregnancy is a must to prevent neural tube defects in the child. Folate deficiency contributes to the etiology of bronchial carcinoma and cervical carcinoma. Blood Level: Normal folic acid level in serum is about 20 nanogram/ml and about 200 microgram/ml of packed cells. In pregnancy the requirement is increased to 400 microgram/day and during lactation to 300 microgram/day. Folic acid alone should not be given in macrocytic anemia because it may aggravate the neurological manifestation of B12 deficiency. Regular supplementation of folic acid may reduce the incidence of birth defects, cardiovascular diseases and cancers. Toxicity of Folic Acid Doses over 1 mg may cause aggravation of vitamin B12 deficiency and may precipitate nerve damage. Since solubility of folic acid is low, large doses should not be given parenterally, as there is danger of crystallization in kidney tubules leading to renal damage. They are widely used as anticancer drugs, especially for leukemias and choriocarcinomas. Folinic acid (citrovorum factor) is given to rescue the patient from toxicity of methotrexate. William Murphy and George Minot showed that liver therapy is very effective to treat pernicious anemia. Dorothy Hodgkin suggested the structure by X-ray diffraction studies (Nobel prize, 1964). Later Robert Woodward synthesized B12 and proved the structure (Nobel prize, 1965). When sulphonamides are given, such micro-organisms cannot synthesise folic acid and hence their growth is inhibited.
Purchase extra super viagra 200mg. ED Series: What's the best drug for Erectile Dysfunction? Obsidian Men's Health in Tysons Corner Va.