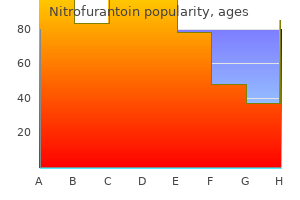
Nitrofurantoin
"Purchase nitrofurantoin 100mg, antimicrobial journals impact factor".
By: H. Benito, M.A., Ph.D.
Medical Instructor, Sidney Kimmel Medical College at Thomas Jefferson University
Viruses in this subgenus are highly oncogenic when injected into newborn hamsters or rats antimicrobial wood purchase discount nitrofurantoin on-line, causing tumours after ca antibiotics for uti prophylaxis 50 mg nitrofurantoin with amex. Viruses in this subgenus are weakly oncogenic when injected into newborn hamsters best antibiotics for acne reviews 100mg nitrofurantoin visa, causing tumours at low incidence after ca antibiotic resistance japan purchase nitrofurantoin 50mg without prescription. These viruses are nononcogenic in newborn rodents, but can cause transformation of rodent cells in vitro; they can cause mild to severe upper respiratory tract disease in young children. The viruses are non-oncogenic in newborn hamsters, but may cause mammary adenomas in newborn female rats and can transform rodent cells in vitro. Infection of animals by adenoviruses may result in (usually mild) respiratory or diarrhoeal illnesses, conjunctivitis, etc. Several animal adenoviruses (11 simian, 2 bovine and 2 canine species) can induce tumours when injected into newborn hamsters. Symptoms may range from mild to an acute inflammatory condition with fever and other systemic effects; the milk contains an increased number of white blood cells, and may contain a significant number of pathogenic microorganisms. Various hypotheses have been proposed to account for maternal inheritance, and different mechanisms may be applicable in different cases. Thus, in certain cases where maternally inherited genes occur in mitochondria, mitochondria from the male parent are presumed to be excluded from the zygote (see. The zygote, which lacks mating potential and mating type characteristics, is designated a/a. These cells therefore synthesize plasmidencoded proteins almost exclusively; the proteins can be labelled by adding. McClung Toabe egg-yolk agar (modified) A medium used for the isolation and identification of clostridia and other anaerobes; it permits detection of lecithinase, lipase and proteolytic activity. Blood (from the heart), or a spleen imprint, is stained with polychrome methylene blue; in a positive test, the stained smear reveals large, blue, squareended bacilli surrounded by a reddish granular capsule. Media etc are placed in the jar, and the lid is secured by means of a screw clamp. The jar is then evacuated, by a suction pump, via one of two screw-controlled needle valves in the lid; this valve is then closed. The jar also has a side-arm to which is attached a small, thick-walled, closed glass vessel which communicates with the interior of the jar and which contains an indicator of anaerobiosis. Transmission occurs by droplet infection or via fomites; the conjunctivae may be important sites of infection. Subsequently, a maculopapular rash develops on the head, then on the limbs and trunk. Viruses are present in the urine, nasopharyngeal secretions etc during the prodromal period and for several days after the appearance of the rash. Measles is usually self-limiting, but may be fatal in young infants or in malnourished children; death is usually due to respiratory complications. Measles immune globulin may be used prophylactically in high-risk patients exposed to measles. Spoilage may involve slime formation, discoloration, souring, and off-odours (due. Even under good hygienic conditions, carcasses are likely to carry 102 105 bacteria/cm2, but spoilage commonly becomes apparent only after bacterial numbers reach ca. After slaughter, the pH of meat gradually falls as the muscle glycogen is converted to lactic acid the final pH depending on the amount of glycogen present at the time of death. Of the spoilage organisms, Pseudomonas spp have the highest growth rate at low temperatures, and this advantage tends to become more marked with decreasing temperature. Vibrio costicola) and species of Acinetobacter and Micrococcus; the micrococci are lipolytic and proteolytic, and some strains can reduce nitrite. Fungi found on chilled carcasses include Cladosporium, Penicillium, Mucor, Rhizopus and Thamnidium, and the yeasts Candida, Cryptococcus and Rhodotorula; these may cause. As well as the factors cited above, the nature of spoilage of packaged meats depends on the type of packaging particularly on the permeability of the packing material to air. Spoilage of chilled red meats typically occurs more rapidly in the presence of oxygen than under vacuum.
An evaluation of the inhibitory influence of cyanuric acid upon swimming pool disinfection antibiotic resistance vietnam purchase nitrofurantoin with visa. Investigations on behalf of the Department of the Environment into the practice of disinfection of swimming pools during 1972 to 1975 antibiotic for strep throat cheap nitrofurantoin 100mg with amex. Pseudomonas dermatitis/folliculitis associated with pools and hot tubs-Colorado and Maine antibiotics for uti not sulfa buy nitrofurantoin with paypal, 19992000 infection xrepresentx lyrics discount nitrofurantoin 50 mg with visa. Microbiological and chemical analyses of indoor swimming pools and virucidal effect of chlorine in these waters. Effects of isocyanuric acid on the polio virus inactivation with hypochlorous acid. Influence of stabilizer concentration on effectiveness of chlorine as an algicide. Inactivation of Cryptosporidium parvum under chlorinated recreational water conditions. Comparative assessment of different biocides in swimming pool water, International Biodeterioration and Biodegradation 2003;51:291-297. Distribution and balance of volatile halogenated hydrocarbons in the water and air of covered swimming pools using chlorine for water disinfection. Thermoregulatory, cardiovascular, and psychophysical response to alcohol in men in 40 degrees C water. Cardiovascular responses during 70 degrees head-up tilt: the effect of elevated body temperature and high alcohol blood levels. The distribution and public health consequences of releases of chemicals intended for pool use in 17 states, 2001-2009. Surveillance data from public spa inspections - United States, MaySeptember 2002. Association between swimming pool operator certification and reduced pool chemistry violations - Nebraska, 20052006. Certified operators: Does certification provide significant results in real-world pool and spa chemistry? Pool chemicalassociated health events in public and residential settings - United States, 1983-2007. Standards for Accreditation of food protection manager certification programs as amended by the 2016 Meeting of the Conference for Food Protection Available at. Ten-year study of pediatric drownings and near-drownings in King County, Washington: lessons in injury prevention. Comparison of exertion required to perform standard and active compression-decompression cardiopulmonary resuscitation. Appendix: table of energy requirements for activities of daily living, household tasks, recreational activities, and vocational activities. Immediate closures and violations identified during routine inspections of public aquatic facilities network for aquatic facility inspection surveillance, five states, 2013. Erosion of dental enamel among competitive swimmers at a gas-chlorinated swimming pool. Caring for our children: National health and safety performance standards; Guidelines for Out-of-Home Child Care Programs, 2nd edition. Prevalence of parasites in fecal material from chlorinated swimming pools - United States, 1999. Trihalomethane comparative toxicity: acute renal and hepatic toxicity of chloroform and bromodichloromethane following aqueous gavage. Volatile disinfection byproduct formation resulting from chlorination of organic-nitrogen precursors in swimming pools. Nontuberculous mycobacteria in aerosol droplets and bulk water samples from therapy pools and hot tubs. Molecular identification of potential pathogens in water and air of a hospital therapy pool. Dust- and endotoxin-related acute lung function changes and work-related symptoms in workers in the animal feed industry.
The Medical College of Georgia and its administrative officers are ultimately responsible for the following: Medical College of Georgia Biosafety Guide- June 2008 1-8 Coordinate efforts between institutional safety and compliance offices to ensure compliance with International antibiotic game nitrofurantoin 50mg free shipping, Federal antibiotic vegetables order 50mg nitrofurantoin with visa, State and Local regulations and guidelines for research and clinical care bacteria worksheet middle school best 50mg nitrofurantoin. Making sure the mechanics and integrity of the facilities are maintained to ensure proper protection of workers within the facilities and proper protection of the environment from hazardous materials within the buildings antibiotics nitrofurantoin generic 50mg nitrofurantoin overnight delivery. Notifying the Biosafety Office, laboratory director and building manager any planned maintenance of the facilities which would require even short suspension of utility operations required to maintain safety in the laboratory. Reporting any issues of non-compliance that are noticed while within laboratories, particularly if these may potentially place Facilities personnel at higher risk for exposure. Because proper design and function of laboratory facilities is one of the key components involved in management of risk within a laboratory, Facility Operations and Maintenance is encouraged to consult and coordinate with the Biosafety Office during design of new or renovated laboratory facilities and/or during construction or maintenance operations which may require untrained personnel to enter areas containing biohazardous materials. Whenever possible, the number of animals requested should be justified statistically. Availability or appropriateness of the use of less-invasive procedures, other species, isolated organ preparation, cell or tissue culture, or computer simulation. Criteria and process for timely intervention, removal of animals from a study, or euthanasia if painful or stressful outcomes are anticipated. These principles were established to safeguard the rights and welfare of human subjects of research investigations, and to fulfill the moral and legal obligations and commitments of the institution. As indicated on the routing form, you "may be subject to criminal, civil or administrative penalties should any of the information contained on this form or in the submitted application. It is the responsibility of each Principal Investigator, Clinical Director and/or Instructional Course Director to ensure their workplaces are in compliance. A copy of the Biosafety Protocol Application form can be found in Appendix A and forms can be accessed online at. Please note that all Biosafety Protocol applications and amendments are reviewed for compliance with the annual training requirements as described in Section 2. The Biosafety Office will also assist in updating information during periodic the biological lab inspections. Amendments can be submitted by altering the original Biosafety Protocol Application forms to include the new information and re-submitting them to the Biosafety Office, or via a fully detailed email to the Biosafety Office. Medical College of Georgia 2-3 Biosafety Guide- June 2008 New personnel should also be offered and provided employee health counseling and/or vaccinations as documented in the Biosafety Protocol prior to initiation of work within the laboratory. This includes changes in inserts, vectors or target cells/tissues/organisms or operations. Similarly, if new operation will be employed with the biological agent(s) which may involve additional risk, this must also be documented. A laboratory assessment by the Biosafety Office will likely be required prior to approval of the amendment. Because major equipment used to contain or handle biological agents, such as Biosafety Cabinets or centrifuges, may not only impact the risk of the protocol, but may also require certification documentation prior to use, such amendments must also be submitted to the Biosafety Office. Biosafety for Basic Research* An Initial Training session is required prior to initiation of work. The "Biosafety for Clinical Research" is a slightly abbreviated, more clinically-oriented version of the more comprehensive "Biosafety for Basic Research" class. The Biosafety Office is more than happy to help "walk" new faculty members through the process and assist them in obtaining any permits or authorizations required to transfer materials to campus. After removal of the biological materials from the laboratory, all surfaces and equipment must be decontaminated using the appropriate disinfection procedures as documented in the laboratory standard operating procedures prior to departure from the laboratory. After removal of all biological materials and decontamination of the laboratory and equipment, the Biosafety Office must be notified to complete the "clearance" process. However, transport of large amounts of biological materials in laboratory moves poses unique challenges. First, laboratory moves are often accomplished with the help of non-authorized personnel. Therefore, whenever possible, equipment containing biological materials should be transported separately by authorized personnel. However, if this is not feasible (due to the size/weight constraints of the equipment), the equipment containing the biological materials should be securely sealed to prevent moving personnel from exposure.
Buy discount nitrofurantoin on-line. ANTIMICROBIAL PROPERTIES OF COPPER.
Diseases
- Listeriosis
- Balantidiasis
- Juvenile dermatomyositis
- Erythroplakia
- 10q partial trisomy
- Circumscribed disseminated keratosis Jadassohn Lew type
- Fetal hydantoin syndrome